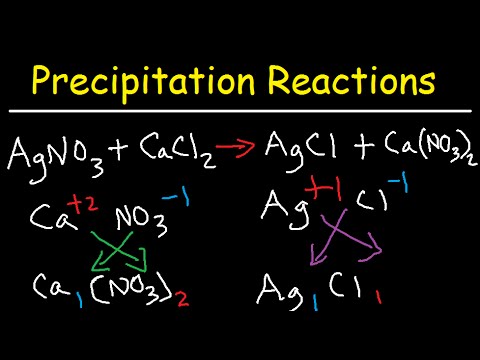
In this video, we're going to focus on precipitation reactions and also write them in ionic equations. Let's consider the reaction between silver nitrate and calcium chloride. The first thing we need to do is determine the products of the reaction. Precipitation reactions are double replacement reactions, where the first and last ions pair up together and the ions in the middle pair up as well. Silver has a +1 charge, indicated by the fact that it pairs with nitrate, which has a -1 charge. Since there is only one nitrate ion, silver must have a +1 charge to neutralize the -1 charge of the nitrate ion. Chloride, like other halides (fluoride, bromide, iodide), carries a -1 charge. Because the charges of silver and chloride ions have the same magnitude, they can be written in a 1 to 1 ratio (AgCl). Alternatively, you can use the crisscross method, which gives us the formula Ag1Cl1 (though we don't need to write the 1). When calcium pairs up with nitrate, the formula produced as a result, using the crisscross method, is Ca(NO3)2. When there are multiple polyatomic ions, they need to be enclosed in parentheses. The other product is Ca(NO3)2. Next, we need to balance the equation. Feel free to pause the video to balance it and then unpause it to see if you have the correct answer. We notice that there are two nitrates on the right side, so we put a 2 in front of AgNO3. We also have two chloride ions on the left, so we need to put a 2 in front of AgCl. The equation is now balanced. Our next step is to write the phases of each substance. Silver nitrate is in the aqueous phase or in the solid phase. Nitrates are always soluble, so anytime you see NO3, it's going...
Award-winning PDF software





Video instructions and help with filling out and completing When Form 8865 Thereof